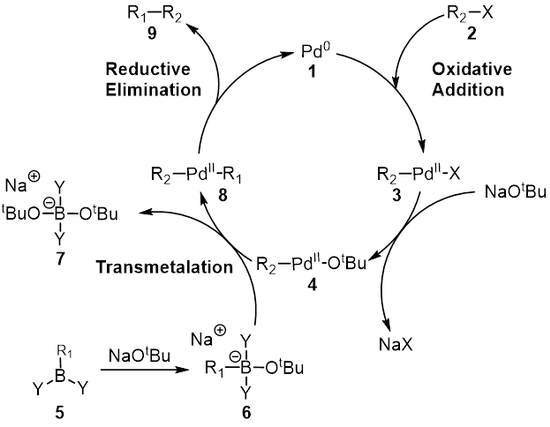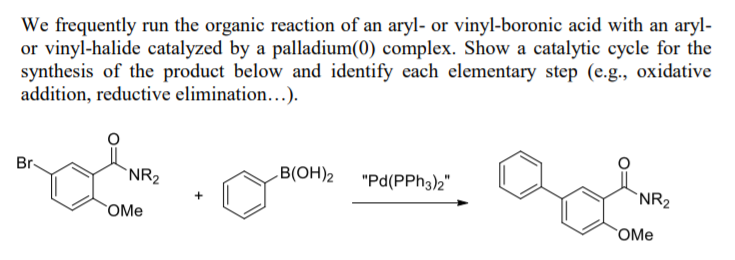
BJOC - Palladium(II)-catalyzed Heck reaction of aryl halides and arylboronic acids with olefins under mild conditions

Table 2 from A palladium-catalyzed three-component cross-coupling of conjugated dienes or terminal alkenes with vinyl triflates and boronic acids. | Semantic Scholar
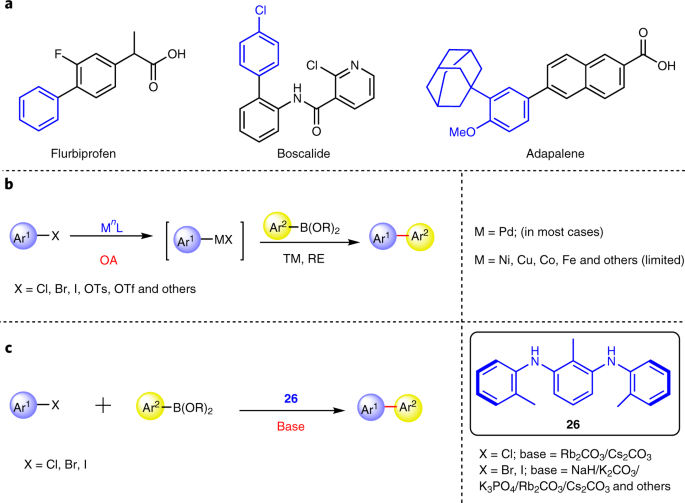
The amine-catalysed Suzuki–Miyaura-type coupling of aryl halides and arylboronic acids | Nature Catalysis

Palladium-catalyzed, direct boronic acid synthesis from aryl chlorides: a simplified route to diverse boronate ester derivatives. | Semantic Scholar

Suzuki–Miyaura Cross‐Coupling Reactions of Alkylboronic Acid Derivatives or Alkyltrifluoroborates with Aryl, Alkenyl or Alkyl Halides and Triflates - Doucet - 2008 - European Journal of Organic Chemistry - Wiley Online Library
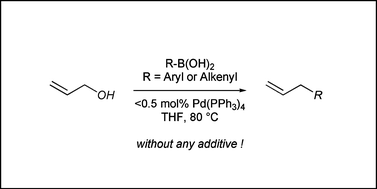
Palladium(0)-catalyzed direct cross-coupling reaction of allylic alcohols with aryl- and alkenylboronic acids - Organic & Biomolecular Chemistry (RSC Publishing)

Suzuki-Miyaura cross-coupling reaction of aryl chlorides with aryl boronic acids catalyzed by a palladium dichloride adduct of N-diphenylphosphanyl-2-aminopyridine - ScienceDirect

Suzuki-Miyaura cross-coupling of phenylboronic acid with aryl halides catalyzed by palladium and nickel species supported on alumina-based oxides - ScienceDirect
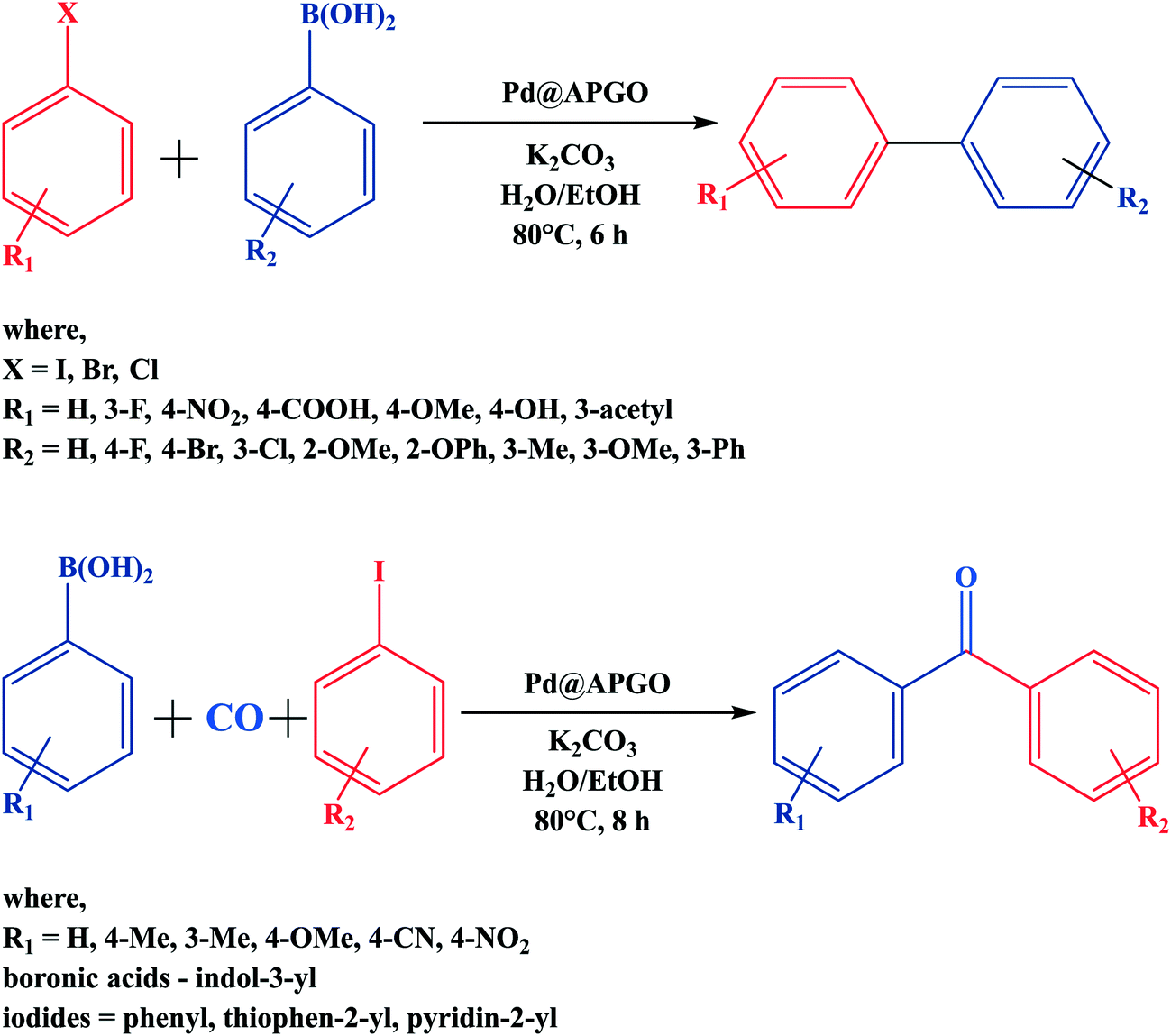
Suzuki–Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis - Catalysis Science & Technology (RSC Publishing)

Fluorinated Aryl Boronates as Building Blocks in Organic Synthesis - Budiman - 2021 - Advanced Synthesis & Catalysis - Wiley Online Library

Scope of the palladium-catalyzed aryl borylation utilizing bis-boronic acid. - Abstract - Europe PMC

SciELO - Brasil - Ionic liquids: a versatile medium for palladium-catalyzed reactions Ionic liquids: a versatile medium for palladium-catalyzed reactions

Table 1 from Fibrous nano-silica (KCC-1)-supported palladium catalyst: Suzuki coupling reactions under sustainable conditions. | Semantic Scholar

Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives